Ionization of Weak Acids and Weak Bases
Ionization of Weak Acids and Weak Bases: Overview
This topic covers concepts such as Degree of Dissociation for Acids and Bases, Ostwald's Dilution Law, Common Ion Effect, Strengths of Acids and Bases, Relative Strengths of Acids and Bases, Strength of Acids, Strength of Bases, etc.
Important Questions on Ionization of Weak Acids and Weak Bases
Explain the reason for the smaller value of higher order ionization constants than the lower order ionization constant of polyacidic bases.
Define polyacidic bases and give some examples.
Ionisation constant of is and concentration of ions is . Then, the initial concentration of is
Which of the following will occur if a solution of a weak acid is diluted to at constant temperature?
The values of formic acid and acetic acid are respectively and . The ratio of acid strength of acids is
Which of the following is a weak alkali?
Define the degree of dissociation of electrolytes.
What is common ion effect? Write an example.
is dissolved in water.
Its base dissociation constant is given by:
Select the weak base from the following:
The increasing order of base strength is
A aqueous solution of a monobasic acid had a freezing point change of What is the for the acid? Given that molal depression constant of is
Arrange in decreasing order of acidity?
(P) 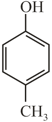 (Q)
(Q) 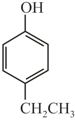 (R)
(R) 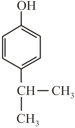 (S)
(S) 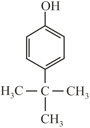
The correct order of increasing in the following aqueous solution is
What is the decreasing order of strength of bases?
The relation between and concentration of the solution of a weak acid can be written as _____.
(A)
(B)
(C)
Enter the correct answer as A, B or C.
If is the ionization constant of a weak acid and is that of the conjugate base, then . If the value of ionic product of water is then the value of _____.
If the acid-base reaction has a , then is the given statement correct or incorrect?
is stronger base than .
If the acid-base reaction has a then the given statement is correct or incorrect?
is stronger acid than .
If the acid-base reaction has a , which of the following statements are true?
(i) is more acidic than .
(ii) is more acidic than .
(iii) and have the same acidity.
